Table 1 Structures and names of 4-chromenone derivatives combined with N-acylhydrazone, and their half-maximal cell growth inhibitory concentration (GI50) obtained from the clonogenic assay, half maximal inhibitory concentrations (IC50) values by in vitro aurora A kinase assay, and logP values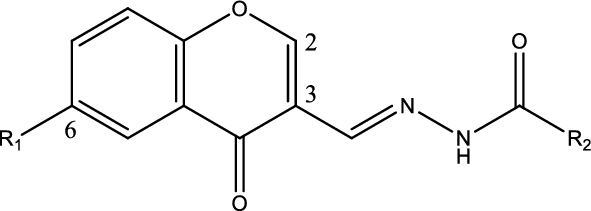

No | R1 | R2 | Chemical Name | GI50 (μM) | IC50 (μM) | logP |
|---|---|---|---|---|---|---|
1 | Chloro | 4-Methoxyphenyl | (E)-N'-((6-chloro-4-oxo-4H-chromen-3-yl)methylene)-4-methoxybenzohydrazide | 82.1 | 27.1 | 2.54 |
2 | Bromo | 4-Methoxyphenyl | (E)-N'-((6-bromo-4-oxo-4H-chromen-3-yl)methylene)-4-methoxybenzohydrazide | 45.0 | 54.1 | 2.81 |
3 | Methoxy | Phenyl | (E)-N'-((6-methoxy-4-oxo-4H-chromen-3-yl)methylene)benzohydrazide | 85.2 | 32.9 | 1.99 |
4 | Methoxy | 3-Bromophenyl | (E)-N'-((6-methoxy-4-oxo-4H-chromen-3-yl)methylene)-3-bromobenzohydrazide | 41.7 | 31.3 | 2.81 |
5 | Methoxy | 3-Fluorophenyl | (E)-N'-((6-methoxy-4-oxo-4H-chromen-3-yl)methylene)-3-fluorobenzohydrazide | 49.5 | 28.8 | 2.14 |
6 | Methoxy | 3-Hydroxyphenyl | (E)-N'-((6-methoxy-4-oxo-4H-chromen-3-yl)methylene)-3-hydroxybenzohydrazide | 36.6 | 1.2 | 1.60 |
7 | Methoxy | 3-Methoxyphenyl | (E)-N'-((6-methoxy-4-oxo-4H-chromen-3-yl)methylene)-3-methoxybenzohydrazide | 61.4 | 34.2 | 1.86 |
8 | Methoxy | 4-Fluorophenyl | (E)-N'-((6-methoxy-4-oxo-4H-chromen-3-yl)methylene)-4-fluorobenzohydrazide | 75.1 | 32.1 | 2.14 |
9 | Methoxy | 4-Methoxyphenyl | (E)-N'-((6-methoxy-4-oxo-4H-chromen-3-yl)methylene)-4-methoxybenzohydrazide | 64.3 | 11.5 | 1.86 |
10 | Methyl | Phenyl | (E)-N'-((6-methyl-4-oxo-4H-chromen-3-yl)methylene)benzohydrazide | 38.2 | 13.1 | 2.60 |
11 | Methyl | 3-Pyridinyl | (E)-N'-((6-methyl-4-oxo-4H-chromen-3-yl)methylene)nicotinohydrazide | 59.2 | 2.8 | 1.26 |
12 | Methoxy | 4-Pyridinyl | (E)-N'-((6-methoxy-4-oxo-4H-chromen-3-yl)methylene)isonicotinohydrazide | 34.8 | 1.4 | 0.65 |
13 | Methyl | 4-Pyridinyl | (E)-N'-((6-methyl-4-oxo-4H-chromen-3-yl)methylene)isonicotinohydrazide | 37.6 | 2.7 | 1.26 |